Imagine a world where waiting for a donor heart is no longer the bottleneck to survival—where a specially designed pump can replace a failing heart and keep you alive and active. That world is inching closer, thanks to a major breakthrough by the French med-tech company CARMAT. Here’s what you need to know: what they’ve done, why it matters, and what the road ahead looks like.
What exactly is this device?
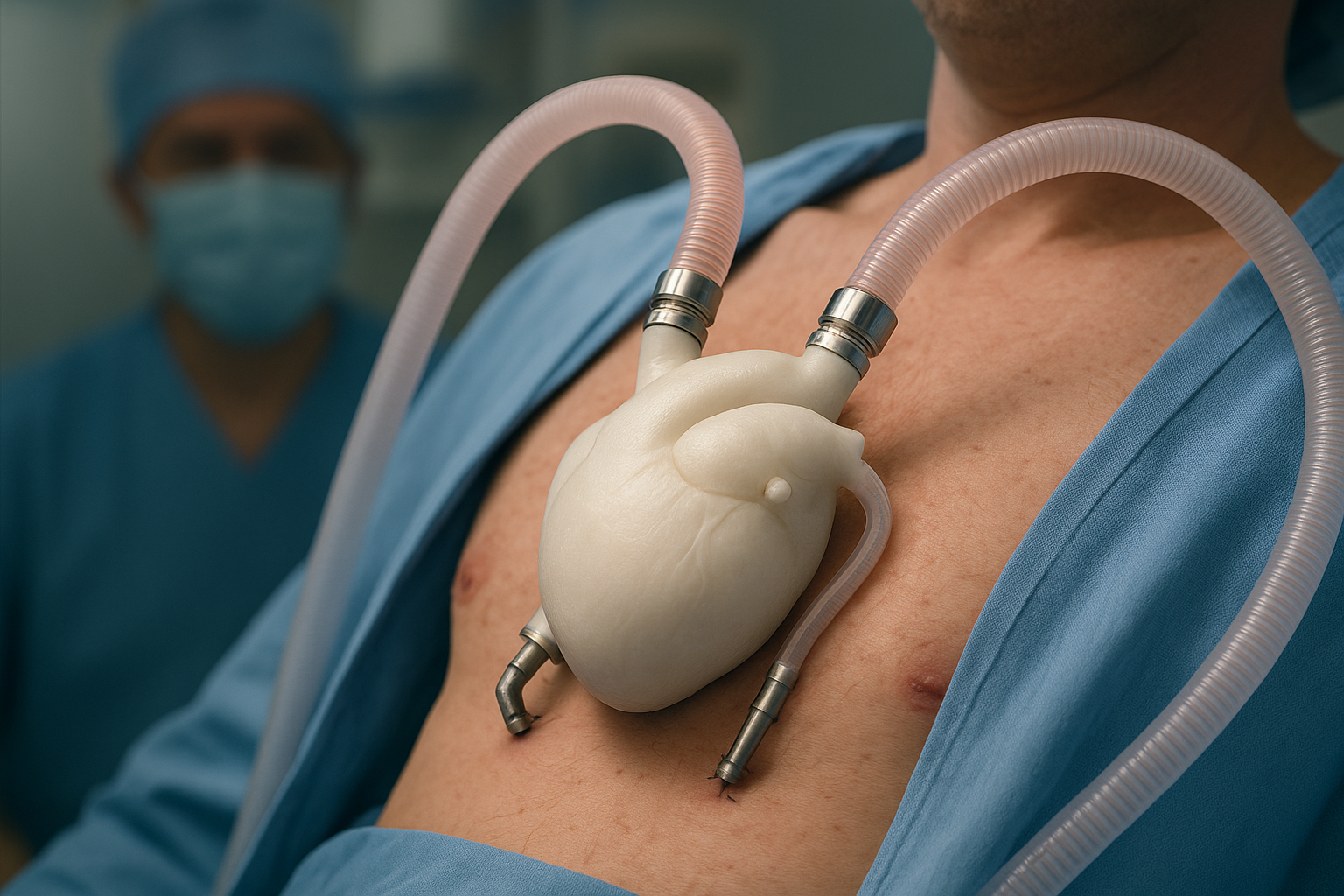
The product is the artificial heart called Aeson®, developed by CARMAT. Several key features make it stand out:
-
It’s a total artificial heart meant to replace both ventricles, not just assist one side of the heart. Cardiac Vascular News+2Smithsonian Magazine+2
-
Designed to mimic a natural heart: It uses biocompatible materials (including bovine tissue) to reduce clotting-and-rejection risks. Smithsonian Magazine+2The Economic Times+2
-
Smart sensors regulate output automatically, adapting pumping strength to the patient’s level of activity (rest vs walk vs stairs) rather than relying on fixed speeds. Oh! Epic+1
-
Received the CE-mark in Europe for the “bridge-to-transplant” indication (meaning it can temporarily replace a heart until a donor arrives) and is heading towards a longer-term solution. The Economic Times+2Cardiac Vascular News+2
In short: it’s not just a mechanical pump—it’s a substitute for the whole heart, with smart regulation and materials that try to live harmoniously inside the body.
What are the milestone achievements so far?
Here are some of the big ones:
-
The first human implant of the Aeson artificial heart occurred in December 2013 in Paris (Hôpital Européen Georges‑Pompidou) in a 75-year-old patient. Smithsonian Magazine+1
-
On February 7, 2025: CARMAT announced the 99th and 100th implants of the Aeson heart during its EFICAS clinical study in France (at Lille and Dijon university hospitals). Carmat+1
-
The latest press release (May 12, 2025) says the EFICAS study (involving 52 patients) has completed enrolment and French authorities approved 21 additional implants while reimbursement discussions are underway. Carmat
So yes: this is real, it’s happening, and it’s rapidly scaling.
Why this matters — and why it could change the future of healthcare
1. Donor heart shortage
One of the biggest constraints in heart failure treatment is donor availability. Many patients die while waiting. A device like Aeson could radically reduce dependence on donors. Cardiac Vascular News+1
2. Longer-term implantability
Earlier artificial hearts were often temporary, used while waiting for a transplant. Aeson is intended as a more permanent solution for end-stage biventricular heart failure (both sides of the heart failing). The Economic Times+1
3. Quality of life & mobility
Because the heart adjusts output automatically, users have potential to return to more normal life activities. One article noted it restores mobility, autonomy and even work potential for patients who thought their days of normal life were over. Oh! Epic
4. Healthcare system impact
Replacing or reducing need for transplants could shift costs, waiting lists, resource allocation, and hospital burden. Countries with scarce donor supply might especially benefit.
The caveats: What isn’t perfect yet
-
While 100 implants is a great milestone, it’s still relatively small compared with the population of patients who might need this therapy.
-
Long-term durability: the device aims at long-term use, but full multi-year survival and complication profiles are still being gathered.
-
Cost and reimbursement hurdles: The May 2025 press release mentions filing for reimbursement in France by early 2026. Carmat
-
Patient eligibility: Not every patient will qualify, given anatomical, physiological or comorbidity constraints.
-
Ethical/regulatory oversight: As with all breakthrough medical devices, widespread deployment needs rigorous oversight, long-term data and safeguards.
What this means for future healthcare (and what to watch)
-
Broader implant indications: As data matures, expect more patients, more hospitals adopting the device, possibly outside of France/Europe.
-
Global access & equity: If successful, similar devices could reach countries with donor shortages (including parts of Asia, Africa, Latin America) and reduce fatal waits.
-
Cost-/value-driven decisions: Payers will ask: is this device cost-effective compared with transplant + lifetime meds? Data from EFICAS will be key.
-
Incremental device evolution: The current device may be the first generation. Next-gen versions might be smaller, wireless-powered, less invasive, or have even longer lifespans.
-
Shift in transplant paradigm: Over time, heart transplantation might become less of a default “only option” and more of one of several options (device vs donor heart) depending on the patient.
-
Ethical/regulatory frameworks adapting: With ever-more advanced implants, regulation around artificial organs, lifespan extension, implant ethics will evolve.
Key reference list (for your blog post or deeper reading)
-
CARMAT press release: 100 implants of Aeson documented on Feb 10 2025. Carmat+1
-
The first implant in 2013, background of the device design. vinnews.com+1
-
Enrollment completion of EFICAS study & regulatory steps. Carmat
-
Overview article on artificial heart design/impact. Oh! Epic